Genetic Models of Rare Diseases
Zebrafish are a remarkably versatile platform for studying rare diseases (RD). The Berman lab studies genetic lesions that cause limb-girdle muscular dystrophy (LGMD). LGMD are a group of RDs that cause weakness and wasting of the muscles most proximal to the trunk, with multiple subtypes and different inheritance patterns that include mutations in LMNA, CAPN3, and DYSF genes. To assess zebrafish phenotypes, we use the birefringence assay and functional assays depending on the disease being modeled. Zebrafish models of LGMD and other muscular dystrophies have been generated to perform drug screens with chemical libraries to identify promising therapies.
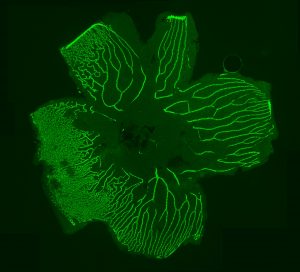
The Berman lab also studies familial exudative vitreoretinopathy (FEVR), a rare congenital disorder commonly caused by mutations of the FZD4 gene and other genes coding for proteins in the Norrin signaling pathway. FEVR is characterized by a lack of blood vessel growth to the periphery of the retina causing leakage and hemorrhage and resulting in retinal detachment and blindness. We have generated a visually impaired zebrafish FEVR model exhibiting abnormal retinal vasculature to study vascular biology in retinovascular disorders and improve patient outcomes.
Lastly, CHARGE syndrome is linked to autosomal-dominant mutations in the CHD7 gene and results in multiple physiological and structural abnormalities, including heart defects, hearing and vision loss, and gastrointestinal (GI) problems. Our zebrafish CHARGE models have allowed us to examine GI structure, innervation, and motility, along with heart rate and responses to anesthesia. These zebrafish models help us to better understand the biological and genetic causes of pathology in these diseases and provide a platform for high-throughput drug screening.
 Xenotransplantation Models of Pediatric Cancers
Xenotransplantation Models of Pediatric Cancers
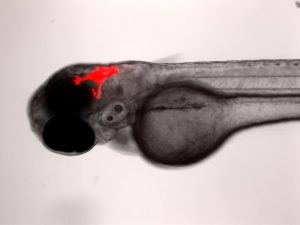
The Berman lab has pioneered the transplantation of human cancer cells into the larval zebrafish for novel drug discovery and improving current clinical chemotherapies. We transplant both human cancer cell lines and patient derived tumour cells into larval zebrafish to better understand cancer progression and to find more personalized therapies. Cells can be tracked in real-time for proliferation, migration and homing to tissue niches. Responses to therapeutics added to the larval water or administered by intraperitoneal injection or gavage can be evaluated by fluorescence microscopy and quantified using ex vivo cell enumeration.
This platform has already been adapted to study metastasis in sarcoma; test novel compounds in AML; and reveal drug response-tumour genotype correlations in primary patient-derived T-cell acute lymphoblastic leukemia (ALL). This model has the potential to provide relevant drug response data in a clinically actionable timeframe, which can help improve patient outcomes and aid in a better quality of life. The Berman lab has leveraged this platform in the Model Systems Node of the Terry Fox Research Institute PRecision Oncology For Young peopLE (PROFYLE) program, which is an innovative pan-Canadian medicine program that strives to create new therapeutic inroads for children and youth with refractory, relapsed, or metastatic childhood cancers. The PROFYLE program aims to molecularly characterize 450 hard-to-treat cancers from children across Canada, experimentally model these tumours, and find molecularly targeted and biologically active treatments.
Genetic Models of Cancer and Cancer Predisposition Syndromes
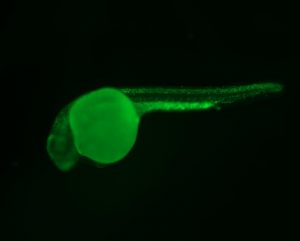
The Berman lab uses transgenic and CRISPR based approaches in the zebrafish to model cancer mutations and cancer predisposition syndromes to better understand the molecular mechanisms underpinning childhood leukemia and other cancers. We have developed and study zebrafish models of Li-Fraumeni Syndrome (LFS), a devastating cancer predisposition syndrome most frequently caused by autosomal inheritance of germline mutations in the TP53 gene.
We also study and have generated models of inherited bone marrow failure syndrome (IBMFS), which are characterized by deficient hematopoiesis in a subset of blood lineages and predispose children to developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Shwachman-Diamond syndrome (SDS) is a well-known IBMFS, commonly caused by germline mutations in the SBDS gene and less frequently in the DNAJC21 gene. Affected children have an increased risk of developing MDS and AML.
We have been developing a model of DNAJC21-deficient SDS. Infant leukemia (IL) is a rare and aggressive leukemia that progresses rapidly with significant treatment and management challenges. Reciprocal translocations of the MLL1 (KMT2A) gene are present in most cases of either infant AML or infant acute lymphoblastic leukemia (ALL). We have generated the first IL zebrafish model by expressing MLL1 gene fusions in pathologically relevant blood cells. Our goal for these models is to increase the biological understanding of these diseases and to perform high-throughput phenotype-based in vivo testing of small molecules and targeted therapies that ameliorate disease and would otherwise be infeasible in other model systems.