Lab Members

Dr. Jason Berman
CEO and Scientific Director of the CHEO Research Institute and the Vice President Research at CHEO
Full Professor, Department of Pediatrics, University of Ottawa
Jason completed a clinical fellowship in Pediatric Hematology/Oncology at the Boston Children’s Hospital and his post-doctoral training at the Dana-Farber Cancer Institute funded by the prestigious Pediatric Scientist Development Program. Jason was recruited to Dalhousie University and the IWK Health Centre in 2005 as the named MSC Clinician-Scientist in Pediatric Oncology. Over the next 14 years, he ascended through the academic ranks to become Professor of Pediatrics, Microbiology & Immunology and Pathology at Dalhousie University and Associate Chair Research for the Department of Pediatrics. In 2017, he assumed the role of interim Vice President Research, Innovation and Knowledge Translation at the IWK Health Centre. He relocated to Ottawa in 2019 to assume the role of CEO and Scientific Director of the CHEO Research Institute and the Vice President Research at CHEO. He is a full professor in the Department of Pediatrics at the University of Ottawa.
Read More… Jason’s research program investigates mast cell transcriptional regulation, personalized leukemia therapy, key transcription factors and cytokines in cancer progression and metastasis, bone marrow failure syndromes and hereditary disorders. He has held funding from CIHR, the Nova Scotia Health Research Foundation, Genome Canada, Ewing Cancer Foundation Canada, Terry Fox Research Institute, Canadian Cancer Society Research Institute, Leukemia & Lymphoma Society of Canada, and C17 – Canadian Children’s Cancer & Blood Disorders Network. Jason aims to translate findings from the laboratory to the clinic. He has been a member of the Children’s Oncology Group Myeloid Committee since 2008, vice-chair of Myeloid Biology (2009-2012) and is the current co-chair of AAML1531, an international trial of risk-stratified therapy in Down syndrome myeloid leukemia. Jason is on the Executive Committee for the pan-Canadian PROFYLE program, where he co-chairs the Model Systems Node. This first-in-Canada program aims to use genomic profiling coupled with functional readouts in model organisms to help identify novel personalized therapeutic options for children, adolescents and young adults with hard-to-treat cancers who have run out of conventional treatment options.

Jennifer Fiene
Graduate Student
Jen is a MSc. student who joined the Berman lab in 2023. She received her B.Sc. earlier that year, after completing the Molecular Biology and Genetics program at the University of Guelph. During her undergraduate thesis project, she worked in the lab of Dr. Terry Van Raay, where she used zebrafish as a model organism to examine the effects of Autism Spectrum Disorder-derived gut microbiome metabolites on development. She really enjoyed working with zebrafish and is excited to continue learning about their potential as an animal model in the Berman lab! Her project involves the investigation of TP53 point mutations associated with the cancer predisposition disorder, Li-Fraumeni Syndrome. When she’s not in the lab, Jen enjoys ice skating, crochet, baking, and spending time with her friends.

Lissandra Tuzi
Graduate Student
Lissandra is a MSc. student in the Department of Cellular and Molecular Medicine. She began in the lab during the Summer of 2022 as a co-op student, and stayed on as a research student for the 2022-23 school year.
Lissandra first started working on the Li-Fraumeni Syndrome (LFS) project with Kim to investigate the roles of TP53 gene mutations on cancer development, but has since moved to the xenotransplantation team to use zebrafish as a model to study cutaneous T cell lymphoma (CTCL) for her Master’s. Lissandra moved to Ottawa from Whitby in 2018 to pursue her BSc. in Biomedical Sciences, which she completed in May 2023. She loved Ottawa (and the Berman Lab) so much that she decided to stay for a Master’s. When she is not hanging out with her fish, she enjoys hiking, crafty things, trying new dinner spots, and meeting new people!

Sergey Prykhozhij
Research Associate
I am using the zebrafish animal model to create disease models and gain
insight into the mechanisms of rare genetic diseases, blood-related disorders and cancer. Gene editing using CRISPR/Cas9 is currently the main tool for this purpose and I have contributed significantly to this area of zebrafish disease modeling by developing computational and experimental tools as well as by writing review articles on the subject. I am currently working on several muscular dystrophy projects in partnership with AGADA Biosciences, where we developed new muscular dystrophy models. My second area of research is on cancer models involving Li-Fraumeni syndrome mutations in tp53 gene as well as its modifier gene mutations. I have also been involved in creating and characterizing zebrafish disease models of blood disorders and several other rare genetic diseases.

Nicole Melong
Researcher
Nicole joined the Berman lab group in February 2014 at the IWK Health Centre/Dalhousie University in Halifax, NS. She moved with the Berman lab to the CHEO RI/University of Ottawa in September 2019.
Nicole uses the larval zebrafish as a xenotransplantation platform for drug discovery and improving current clinical chemotherapies for pediatric cancers. She is especially interested in using this platform as patient avatars for those with hard-to-treat cancers and few treatment options left. This platform can provide rapid pre-clinical drug response data for relapsed, refractory, or metastatic tumours, to the oncology staff and can provide a personalized medicine aspect that is not feasible in other models. Nicole is enjoying Ottawa and in her spare time likes to check out local restaurants, cook, watch sports, and hang out with friends and her dog Lloyd.

Nadine Azzam
Research Coordinator
Nadine joined the lab in 2020. She uses the larval zebrafish as a xenograft model to advance drug discovery and enhance existing clinical chemotherapies for pediatric cancers. Her primary focus lies in using this platform to create patient avatars for young patients with high-risk cancers with limited treatment options. This model has the potential to provide relevant drug response data in a clinically actionable timeframe, which can help improve patient outcomes and aid in a better quality of life. Outside the lab, Nadine likes to play the piano, volleyball, and travel.

Kim Kobar
Graduate Student
Kim is a MSc. student in the Department of Cellular and Molecular Medicine, and she is using a zebrafish model to investigate the roles of TP53 and modifier gene mutations in Li-Fraumeni Syndrome as well as to examine genetic polymorphisms that result in a predisposition to cisplatin-induced ototoxicity. Kim is originally from northern Manitoba, but moved abroad to obtain her B.Sc in Biology and Chemistry from the University of Wisconsin-Superior where she studied the roles of transcriptional co -regulators in both normal development and rhabdomyosarcoma using a zebrafish model. Outside of the lab, Kim enjoys playing hockey and soccer, adventuring in nature with her dog Ember, playing the guitar, and exploring Ottawa with her friends

Kevin Ban
Research Technician
Kevin graduated from the University of Ottawa in 2016 with a BSc. in Biology. After graduating, he spent 3 years working as a technician in Dr. David Dyment’s lab at the CHEO Research Institute using zebrafish to study epilepsy related rare diseases. Kevin joined the Berman Team in January 2020 and is excited to continue to build upon his skills to further learn the possibilities that zebrafish have to offer.

Sarada Ketharnathan
Postdoctoral Fellow
Sarada received her Bachelor’s degree in Industrial Biotechnology from SASTRA University, India. Having developed a liking for molecular biology and genetics, she pursued a Master’s degree in Human Genetics from SRMC, India and graduated with a gold medal in 2014. Sarada then moved to New Zealand for her PhD in the Horsfield lab at the University of Otago where she used zebrafish to study two different diseases. She established a pipeline for the functional characterization of gout-associated genetic variants and in parallel, studied the role of cohesin in normal embryonic hematopoiesis and leukemia. Having enjoyed a fair share of Antarctic winds, she moved to Canada in 2020 where she joined the Berman lab as a postdoctoral fellow. She is currently investigating the pathogenesis of bone marrow failure syndromes and their evolution to leukemia. Outside of the lab, Sarada enjoys travelling, hiking, yoga and experimental cooking.

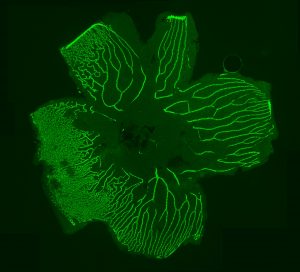
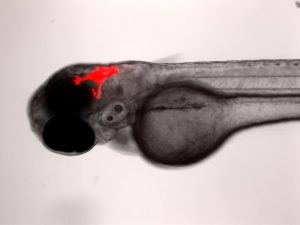 Xenotransplantation Models of Pediatric Cancers
Xenotransplantation Models of Pediatric Cancers